IP Agreement Flowchart
Contracts Guide
Disclose Your Invention
MTA/CDA Assistance
COVID-19 Related Agreements
Main Campus Research
Office of Sponsored Research
Education Links
Connect with Industry
Technologies for Licensing
Industry Collaboration Opportunities
Research and Clinical Trials
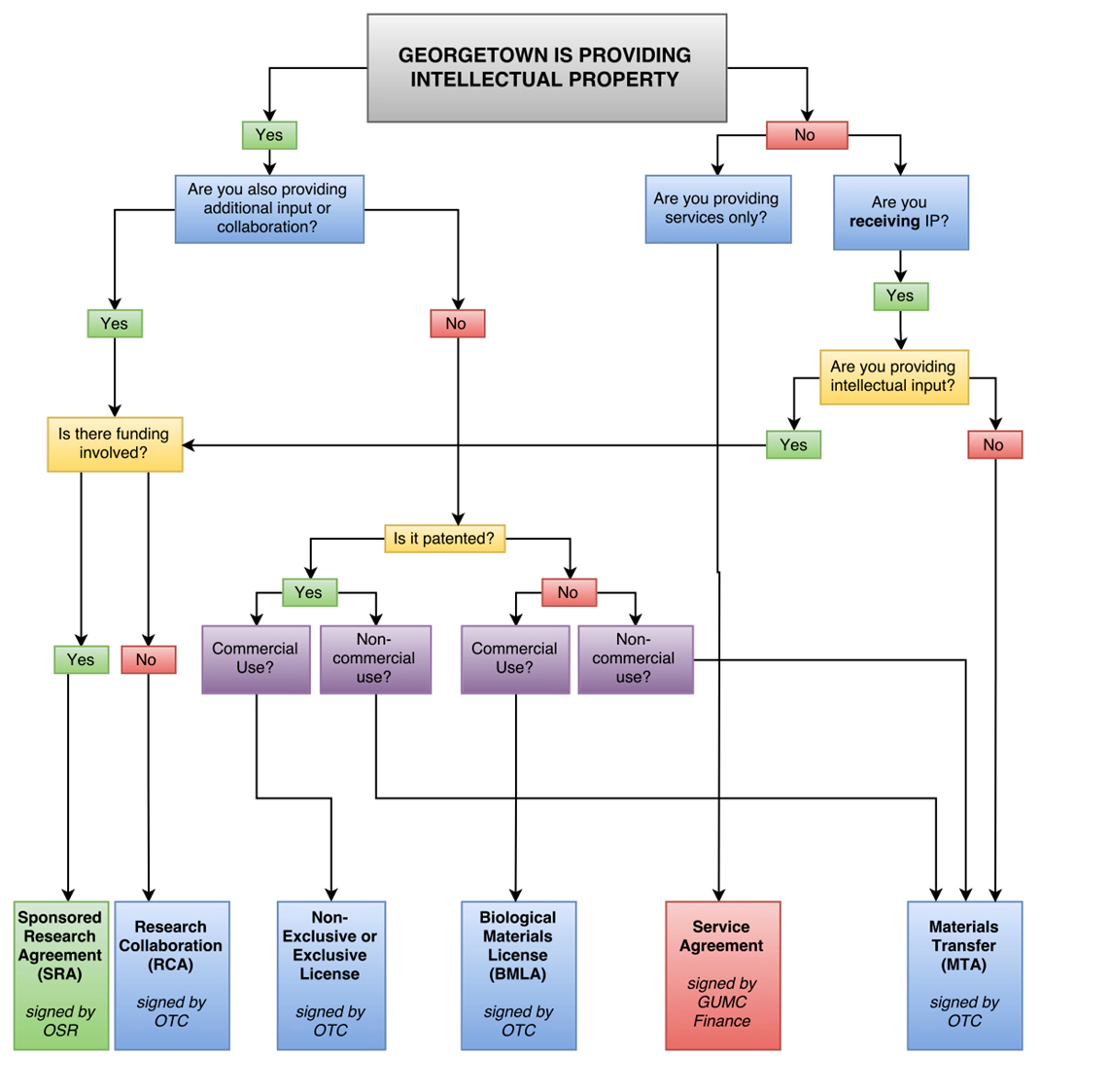
Material Transfer Agreements (MTA): A material transfer agreement is between two parties for the transfer of materials from one party (provider) to another (recipient) for the recipient’s independent research. This agreement is a form of a non-exclusive license from provider to recipient for limited internal research use. The recipient usually grants provider joint publication rights (e.g., co-authorship), as scientifically applicable. As between non-profit to non-profit, there is usually no intellectual property reach-through (e.g., the party that develops intellectual property owns it). However, where a Company is the provider (e.g., of a drug) and provides the MTA to Georgetown as recipient, the Company attempts to obtain intellectual property rights on any new uses or developments related to its material while Georgetown University does its best to negotiate the language to retain rights in intellectual property developed by its faculty.
Research Collaboration Agreement (RCA): A research collaboration agreement is usually between two non-profit institutions where one or both parties exchange materials and/or collaborative research services with a shared common goal and shared intellectual property interests. Both parties contribute to the research aims, unlike the MTA, which is independent research by the recipient. In the event of any new intellectual property conceived or developed under this type of agreement, such intellectual property in most cases belongs to the inventing university. If the intellectual property is jointly owned between the institutions, then the parties typically endeavor to enter into an inter-institutional agreement to jointly manage the intellectual property.
Sponsored Research Agreement (SRA): A sponsored research agreement is between two parties with monetary funding from one party (the sponsor) to the other (the recipient, GU) to conduct research that has a shared common research goal between the parties. In this type of agreement the funding party, typically a company, relies on the intellectual input of the university investigator to meet the goals of research. Georgetown University will typically own the intellectual property that arises from sponsored research and will grant the funding party a limited research license as well as an option to negotiate an exclusive commercial license to make and use such intellectual property.
Biological Materials License Agreement (BMLA): A biological material transfer agreement is an agreement between two parties e.g., Georgetown University and a company, whereby Georgetown University provides valuable, unpatented research tools (e.g., transgenic mice, modified cell lines, antibodies, etc.) to a company for company’s internal non-commercial research purposes, in exchange for a negotiated license fee.
Independent Contractor/Service Agreement: An agreement between two parties where one party contracts the other party for a fee to conduct unique or special services that do not result in the new development of jointly owned inventions, but rather provide a service for mechanical “hands-on” work, e.g., running already-established assays and providing results with little to no interpretation or thoughtful analysis. In these agreements, the service provider does not usually have a stake in any intellectual property or “deliverable” (e.g., the data or results) directly resulting from its services.
License Agreements: A license agreement is between Georgetown and another entity, typically a company, for the explicit commercial use of technology (to make, have made, use, and sell). The agreement may be exclusive, non-exclusive, or a time-limited option, for the defined use, development and/or sale of the patented (pending or issued) and licensed intellectual property. These types of commercial license agreements, which include defined research or technical milestones, payments, fees and royalties on sales, and other intellectual property and patent prosecution matters are negotiated on a case by case basis.
Data Use Agreement (DUA): These agreements involve the transfer of a Limited Data Set (LDS), which under 45 C.F.R Section 164.514e(2) is defined as protected health information that excludes the following direct identifiers of the individual or relatives, employers or household members of the individual :
- Names
- Postal address information (other than town or city, state, and zip code)
- Telephone numbers
- Fax numbers
- Electronic mail addresses
- Social security numbers
- Medical record numbers
- Health plan beneficiary numbers
- Account numbers
- Certificate/license numbers
- Vehicle identifiers and serial numbers (including license plate numbers)
- Device identifiers and serial numbers
- Web universal resource locators (URLs)
- Internet protocol (IP) address numbers
- Biometric identifiers, including fingerprints and voiceprints
- Full-face photographic images and any comparable images
But a LDS may include a limited set of personal identifiers such as: city, state, zip code, elements of date, and other numbers, characteristics, or codes not listed as direct identifiers set forth above.
A DUA allows the sharing of a LDS with another entity not associated with the study or the researcher’s institution for defined research purposes and with safeguards included in such agreement to ensure against unauthorized uses or disclosures of the LDS.
Note: a DUA is not required to transfer data if the recipient is included in the IRB Authorization or the IRB has waived the authorization requirement. However, in any situation involving a data transfer, it’s best practice to remove as many identifiers as possible that still allows the research to be performed. If all identifiers have been removed (including dates and zip codes) and the information is coded, then the data would be considered completely de-identified and there is no need for a more stringent and protective DUA.
Please contact our office if you have further questions via email (techlicensing [at] georgetown [dot] edu) or phone (202-687-0881). Also, please note that the Office of Technology Commercialization is the authorized signatory to bind the University to intellectual property contracts.