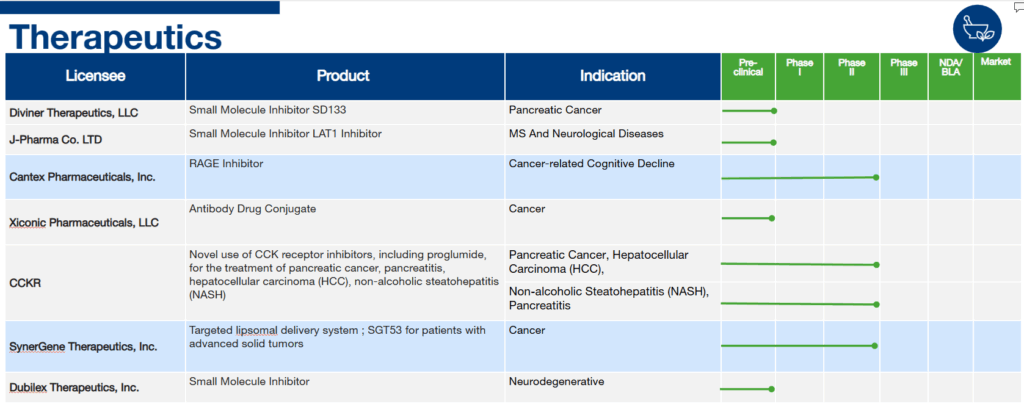
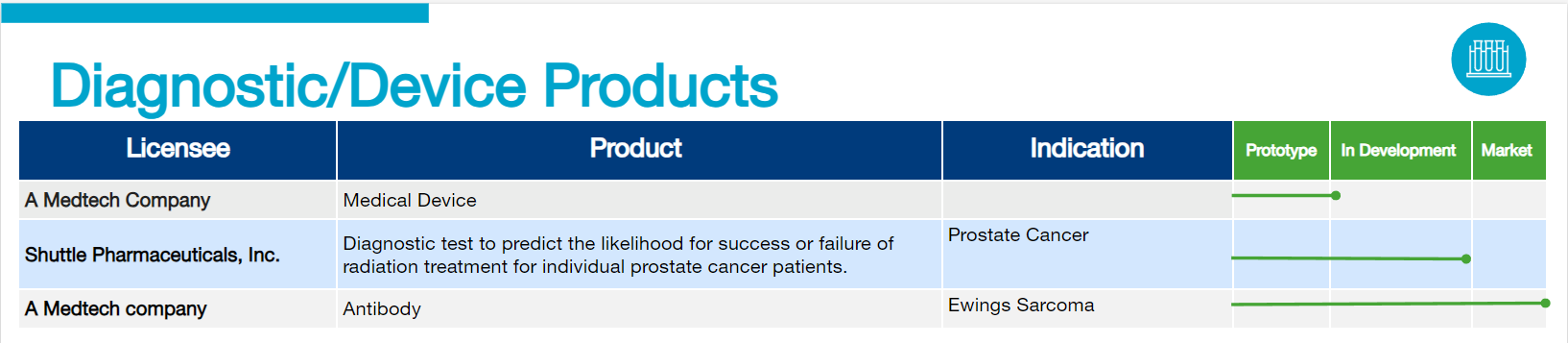
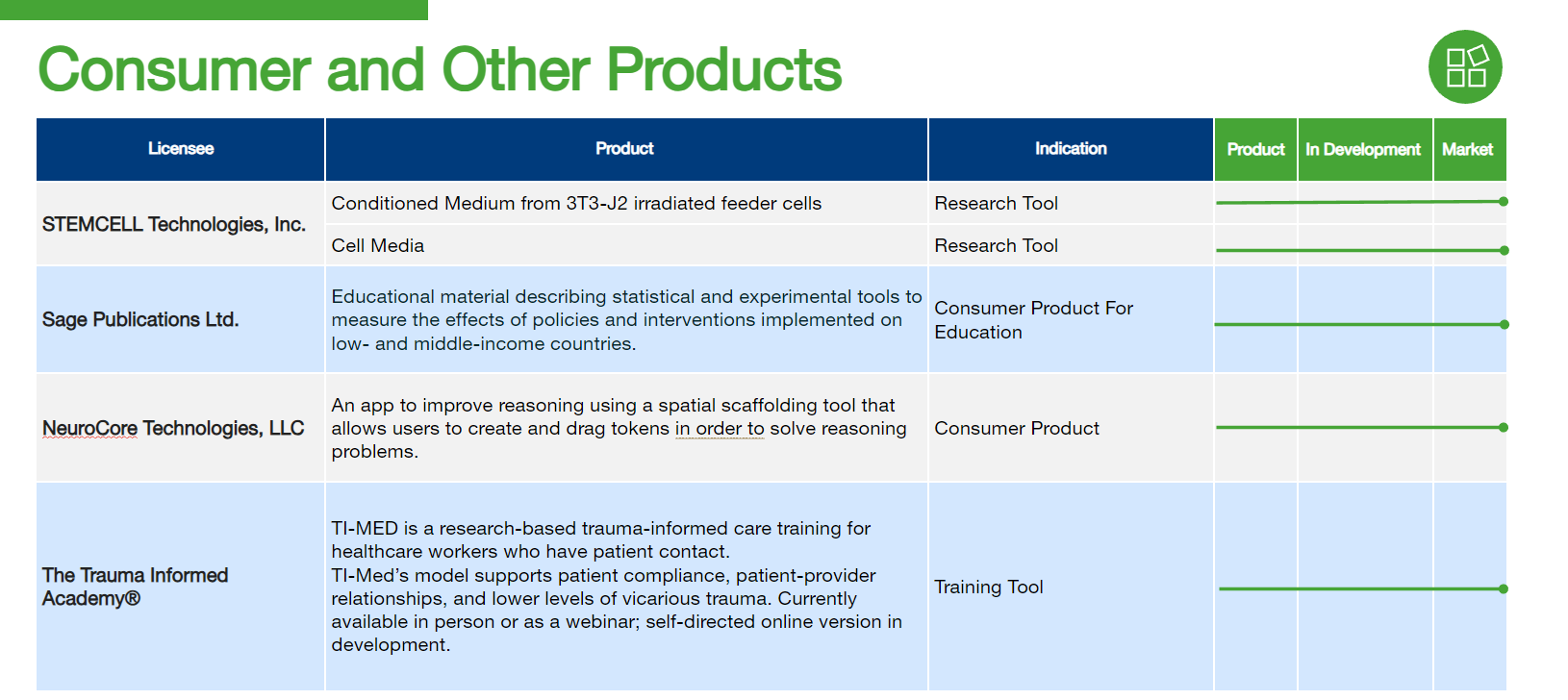
Georgetown’s Technology Pipeline
At Georgetown University, innovation thrives. Our Technology Pipeline showcases exciting technologies under development by industry partners who have licensed Georgetown’s discoveries and innovations. This pipeline represents examples of our groundbreaking research being translated into real-world applications, highlighting the university’s commitment to collaboration and technological advancement across various sectors.